Abstract
Background: Peripheral blood biomarkers of tumor microenvironment and immune surveillance such as absolute lymphocyte (ALC) and monocyte (AMC) counts are independent prognostic factors in several hematologic malignancies including multiple myeloma. The timing and prognostic impact of immune reconstitution has been studied after autologous hematopoietic stem cell transplantation, less is known about its significance in newly diagnosed multiple myeloma prior to transplantation.
Methods: We studied 771 patients with newly diagnosed multiple myeloma who were treated with novel agents at Mayo Clinic between 01/2004 and 12/2015. Peripheral blood absolute lymphocyte and monocyte counts were measured at the time of treatment initiation and one month thereafter in all patients (including data obtained between 14 and 42 days after treatment initiation). The outcome of interest was overall survival. The peripheral blood parameters of interest were abnormal ALC (reference range 800-2400/µL) and AMC (reference range 500-1500/µL) at baseline and at one month. Immune dysregulation was defined as both abnormal ALC and AMC. Immune reconstitution was defined as recovery of both normal ALC and AMC. The Wilcoxon signed-rank test was used to compare the peripheral blood parameters before and after the first month of treatment. Multivariable-adjusted proportional hazards regression models were used to assess the associations between changes in peripheral blood parameters and overall survival. P-values below 0.05 were considered statistically significant.
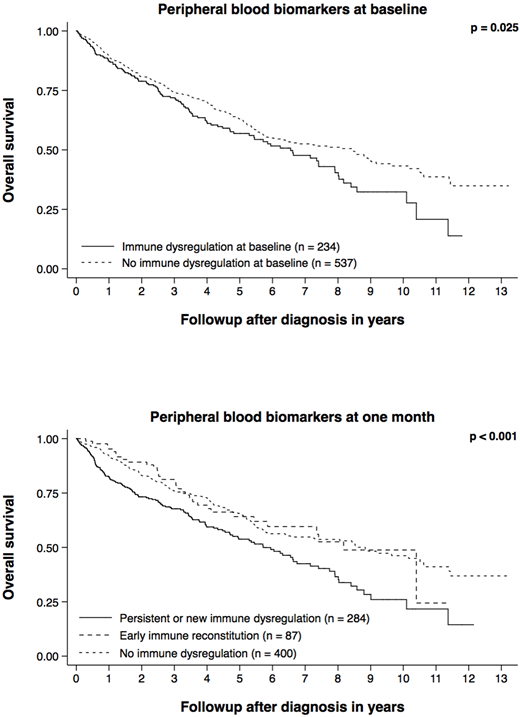
Results: The median age at diagnosis was 65 years (27 - 90) and 459 patients were male (60%). The three most common first-line regimens were lenalidomide + dexamethasone, bortezomib + cyclophosphamide + dexamethasone, and bortezomib + lenalidomide + dexamethasone. Two hundred and eighty patients (36%) went on to undergo autologous hematopoietic stem cell transplantation as part of their first-line therapy. The median ALC decreased from 1100/µL (range 60 - 5590) at baseline to 850/µL (range 60 - 5590) at one month (p < 0.001). The median AMC increased from 330/µL (range 0 - 1840) at baseline to 420/µL (range 0 - 1840) at one month (p < 0.001). The median time between re-assessment of ALC and AMC was 25 days (range 15 - 42). Two hundred and thirty-four patients (31%) had evidence of immune dysregulation at baseline (both abnormal ALC and AMC). Eighty-seven of these 234 patients (37%) recovered normal ALC and AMC at one month (early immune reconstitution). One hundred and thirty-seven of the 537 patients with normal ALC and AMC at baseline (26%) developed new immune dysregulation at one month. The absence of immune dysregulation at baseline (compared to the presence thereof) was associated with better overall survival (HR 0.77, 95% CI 0.61 - 0.97, p = 0.025, n = 771). The absence of immune dysregulation at one month (compared to the persistence or development thereof) was associated with better overall survival (HR 0.63, 95% CI 0.50 - 0.80, p < 0.001, n = 771). Early immune reconstitution (compared to the persistence or development of immune dysregulation) was associated with better overall survival (HR 0.62, 95% CI 0.43 - 0.92, p = 0.016, n = 771). Both associations remained statistically significant after adjusting for age at diagnosis, sex, International Staging System stage, and eligibility for transplantation: HR 0.70 (95% CI 0.54 - 0.90, p = 0.006, n = 612) and HR 0.59 (95% CI 0.39 - 0.90, p = 0.014, n = 612), respectively.
Conclusions: Peripheral blood biomarkers of immune dysregulation vary over time and have prognostic significance both at baseline and during follow-up. The presence or development of immune dysregulation in newly diagnosed multiple myeloma is an independent risk factor. The favorable impact of early immune reconstitution suggests that immune dysregulation is a potentially modifiable risk factor that may be exploited for therapeutic benefit.
Lacy:Celgene: Research Funding. Gertz:celgene: Consultancy; Medscape: Consultancy; janssen: Consultancy; Prothena: Honoraria; Apellis: Consultancy; Ionis: Honoraria; annexon: Consultancy; spectrum: Consultancy, Honoraria; Amgen: Consultancy; Physicians Education Resource: Consultancy; Abbvie: Consultancy; Research to Practice: Consultancy; Teva: Consultancy; Alnylam: Honoraria. Dispenzieri:Celgene, Takeda, Prothena, Jannsen, Pfizer, Alnylam, GSK: Research Funding. Russell:Vyriad: Equity Ownership. Kapoor:Takeda: Research Funding; Celgene: Research Funding. Kumar:AbbVie: Membership on an entity's Board of Directors or advisory committees, Research Funding; KITE: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal